Digital integrity through compliance, auditability and traceability
Complete data transparency, seamless compliance – manage your pharmaceutical production in real time.

End-to-end data transparency for
quality, safety and compliance
Our specialised Orchestra software solution for the pharmaceutical industry brings together data from production, laboratories and quality assurance in a centralised, end-to-end system – for maximum transparency and full compliance along the entire value chain.
In pharmaceutical manufacturing, meticulous documentation and valid data are crucial for product quality, patient safety and regulatory acceptance. Orchestra provides this data in a GxP-compliant, audit-proof and scalable manner.
The advantages of using Orchestra at a glance
- GxP-compliant & ready for validation
Meets essential GxP requirements with electronic signatures, audit trail, role/rights model, change & version control, and auditable & approvable workflows. - 100 % audit trail & batch control
Every change is versioned in a tamper-proof manner – batches can be traced back in seconds. - End-to-end transparency
Links production, laboratory and supply chain data in real time. - Seamless integration
Open interfaces to LIMS, MES, ERP and IoT devices as well as the shopfloor shorten project runtimes. - Secure, scalable architecture
On-premises, private cloud or hybrid – encrypted, highly available, future-proof. - Many years of experience with pharmaceutical customers
In-depth industry knowledge and tried-and-tested best practices from numerous projects.

Use cases in the pharmaceutical industry
Low-code for regulated industries – validation from start to finish
Orchestra is much more than a collection of adapters – it is a complete low-code platform designed specifically for use in regulated environments. From initial modelling in the designer, through versioning, dependency management and automated testing in a validated test environment, to release and provisioning: every step is auditable and fully traceable
This ensures that the validated and quality-assured artefact – for example, an interface – remains unchanged after release. Thanks to configurable reusability, interfaces can be used for different machines or systems without having to redevelop, test and validate them each time. This means scalable, fast and cost-efficient implementations – with consistently high compliance.
Connectivity & Serialisation
Scalable across all levels – from the machine to the enterprise and government level: serialisation meets industrial interoperability.
Our Orchestra solution seamlessly supports serialisation processes across all automation and IT levels (Levels 1 – 5). This creates end-to-end traceability – from data collection on the line to reporting and exchange with external parties.
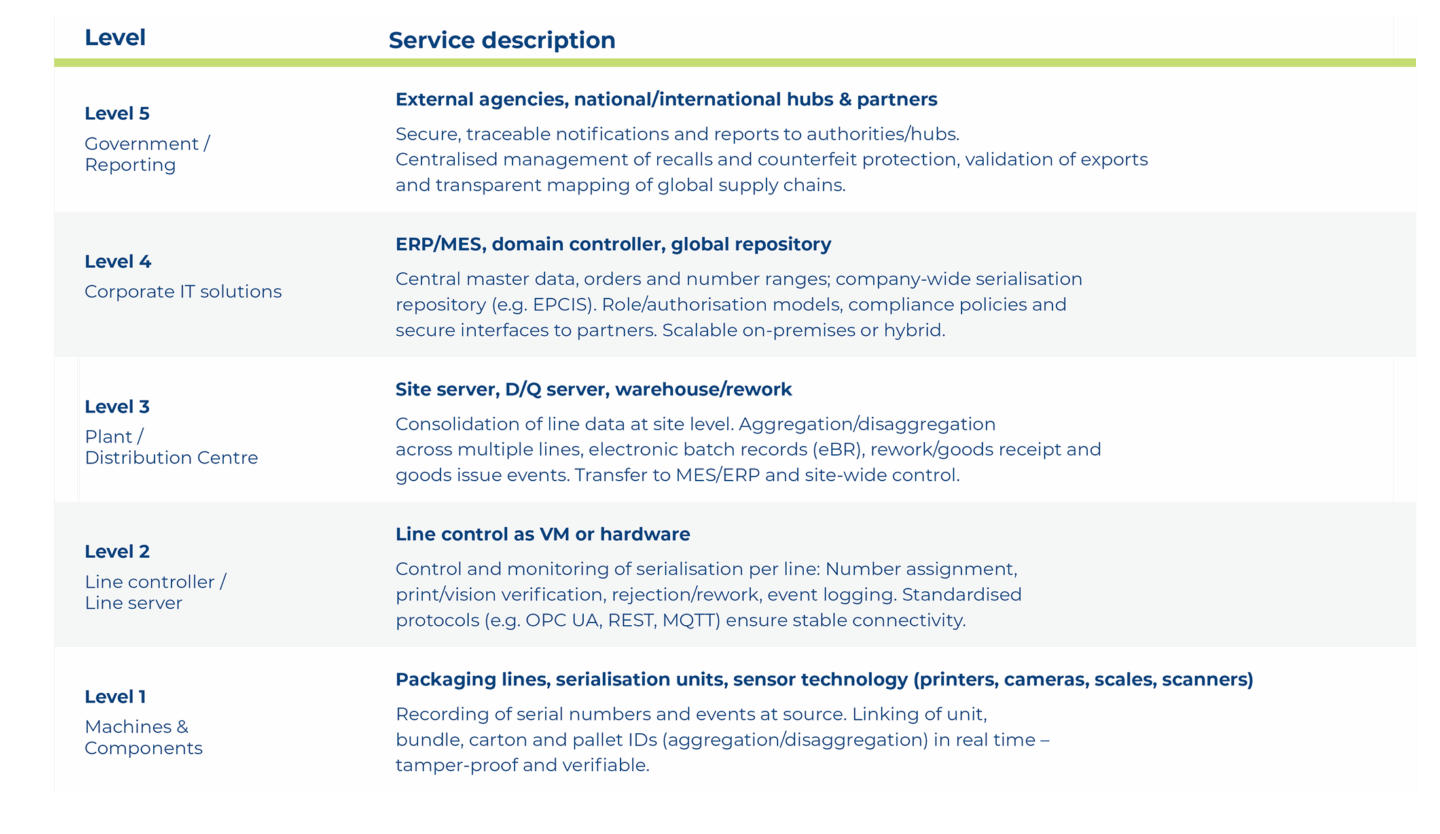
Advantage: A consistent architecture across levels 1 – 5 reduces media discontinuity, prevents isolated applications and forms a robust basis for track & trace processes – regardless of whether they are operated locally, hybrid or cloud-based.
More than just software: your full-service partner for pharmaceutical compliance
With Orchestra, we offer more than just a technical solution – we actively support you:
- Validation support:
Our experts support you in validation in accordance with GxP, GMP and other pharmaceutical standards – with documented methodology and best practices. - First-hand pharmaceutical-compatible processes:
As a German company with years of industry experience, we follow all the relevant standards and certifications – both internally and externally. - 24/7 support from our own team:
No outsourcing, no waiting on hold. You’ll talk to specialists who know your system – around the clock. - Regional, experienced, on equal terms:
We are your partner in the region – with short distances, direct communication and a deep understanding of regulatory and operational requirements in the pharmaceutical industry.

Make your processes audit-ready – every day
In an industry where compliance, safety and efficiency are crucial, our Orchestra software solution is your key to sustainable success. We combine technological excellence with pharmaceutical expertise – for smooth, secure and validated operations.
Discover our solution – practical, proven in the pharmaceutical industry and tailored to your requirements.

References
Uhlmann Pac-Systeme GmbH & Co. KG is a leading global system supplier for the sustainable packaging of pharmaceuticals in blisters, trays, bottles and cartons. In addition to its innovative packaging lines, Uhlmann offers consulting, project management, comprehensive services and digital solutions from a single source. An integration solution based on Orchestra enables Uhlmann Pac-Systeme to centrally connect and control a wide range of machines and systems to the Pexcite platform and creates a basis for clear and easy-to-understand operation of the interfaces due to the low-code approach.
Sarah Blomeier

